
Technical FAQ
General questions
The refractive index of the embedding media for most of our premounted probes is 1.46. The only exception is for our DNA-PAINT probes, where the embedding medium is based on aqueous buffer and subsequently a refractive index of around 1.33 is given.
The ready-to-use probes are embedded in a polymer, which contains antifading reagents.
Yes, in principle we can offer custom solutions with all kind of commercial organic dyes or fluorescent proteins. Please be aware of that changing the fluorophore might influence your experimental results and lead to a deterioration of your data quality. We mainly use ATTO and Alexa Fluor dyes, because they show an extraordinary performance for single-molecule and super-resolution experiments and are widely used throughout the community. If you are interested in a custom solution please contact us under info@gattaquant.com and we will be happy to help.
Yes, we can offer custom probes with distances between fluorescent marks in the range of 6–350 nm. Depending on your specific application you can choose the distance, which works best for you. Please contact us under info@gattaquant.com and we will be happy to help.
Flow chambers are mounted on commercial objective slides with dimensions of 3 x 1 inch2 with 1 mm thickness. The objective slide offers a GATTAquant logo and a sticker label presenting the most important information about your probe, both of them placed on the opposite side of the slide. The size of the coverslip is 22 x 22 mm2, but on request we can also offer 18 x 18 mm2. To fulfill the demands of high performance experiments and to provide the highest slide quality to our customers, we only use Marienfeld High Precision coverslips #1.5 with a thickness of (170 ± 5) µm. When mounting the slide to your microscope, please be aware of that the coverslip points to the objective (e.g. on an inverted microscope the coverslip points down to the objective while the logo and the label remain readable). If you need special coverslips, different dimensions or other custom solutions please contact us under info@gattaquant.com and we will be happy to help.
Nanorulers or beads are immobilized on the inner surface of the coverslip (random and unspecific binding of rulers might occur on the objective slide, too, but should not be imaged!) and show a density of around 0.5–2 rulers/µm2, consequently one can expect hundreds of millions of structures on one slide. They are randomly and homogenously distributed all over the coverslip – aggregations are usually rare. Please be aware of that nanorulers or beads are much smaller than e.g. cell samples, therefore a smaller field of view is recommended (40 x 40 µm2 or smaller).
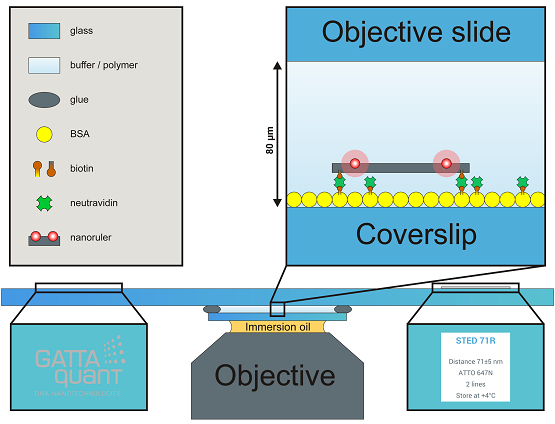
Widefield: Please assure that you placed the GATTA-slide according to your setups specifications on the sample holder with the coverslip facing to the objective. Use an accurate amount of immersion oil (too much oil enhances drift, too less oil makes imaging impossible) and set the objective on a z-value, which allows the oil to touch the coverslip but still offers enough space to approach to the coverslip in z. Using scientific cameras for single molecule acquisition like EMCCD or sCMOS and setting enough laser power one could be able to detect the oil phase by seeing small floating particles (it is not mandatory to detect/see the oil phase). In general we strongly recommend using the camera for approaching (with image dimensions of 40 x 40 µm2 or smaller), approaching via the ocular is very demanding and offers no benefits compared to the camera approach. Further we recommend to set the level of contrast (common scientific cameras use 16-bit grey scale color depth) to a fixed value showing enough contrast in the oil phase instead of using the auto contrast. The auto contrast makes it harder to detect the correct imaging plane. While approaching in z the focus travels first through the oil phase and passes the oil-coverslip interphase (depending on your settings a dim and inhomogeneous dirty surface might be visible). From this interphase it is another 170 µm to the correct imaging plane – the coverslip/embedding media interphase (see also the video in the How-To-Use section). Because the image contrast was set to a fixed value in the beginning, the intensity increases during approaching and maybe oversaturates, please adjust the contrast stepwise and manually. When arriving at the correct focal plane you should be able to detect individual and homogeneous PSFs from the nanorulers or beads. If you experience a focal shift while passing through z or detect elliptic PSFs please clean the coverslip and the objective thoroughly, use fresh oil and approach again. If you experience low S/N, high background and/or slightly distorted looking PSFs you are probably on the wrong imaging plane (e.g. embedding media/microscope slide interphase or oil/coverslip interphase). In the following typical imaging parameters for TIRF applications are given. Laser power: 3–10 mW, EM gain: 50–200, integration time: 30–100 ms. Please be aware of that these parameters strongly depend on your setup and image plane illumination and only represent suggestions.
Confocal: Please assure that you placed the GATTA-slide according to your setups specifications on the sample holder with the coverslip facing to the objective. Use an accurate amount of immersion oil (too much oil enhances drift, too less oil makes imaging impossible) and set the objective on a z-value, which allows the oil to touch the coverslip but still offers enough space to approach to the coverslip in z. Approaching to the imaging plane can be done in different ways, depending on your setup and equipment. If you are using cameras, please take a look at the widefield approach above. For APDs it is recommended to use the ocular. Usually you should be able to detect backreflections from the interphases. The oil/coverslip interphase shows a very dim – in the best case even no – backreflection, due to the similar refractive index. The first strong reflection presents usually the correct imaging plane from the coverslip/embedding media interphase. A second reflection can be found if you approach too far in z, passing by the interphase between embedding media and microscope slide. Please assure that you use the first strong reflection for imaging. If you experience low S/N, high background and/or slightly distorted looking PSFs you are probably on the wrong imaging plane (e.g. embedding media/microscope slide interphase or oil/coverslip interphase). In the following typical imaging parameters for confocal microscopes with stagescan and APD detection are given. Laser power: 1–5 µW, scanning speed: 0.5–2 px/ms, pixel size: 50 nm. Please be aware of that these parameters strongly depend on your setup and image plane illumination and only represent suggestions.
Depending on your specific application it might be useful to order the samples in solution. However in most cases, premounted rulers or beads, which are ready-to-use are likely the better option since you don’t have to take care of surface immobilization, ruler density control, embedding media, and further technical details. Please be aware of that samples in solution demand your expertise and chemical facilities and that immobilizing and buffering requires additional chemicals, which are not part of the product.
Yes, if you would like to have custom buffers or embedding media please contact us under info@gattaquant.com and we will be happy to help.
DNA-PAINT
DNA-PAINT is a localization based super-resolution technique, which uses transient binding of dye-labeled oligonucleotides to the DNA origami structure in order to generate the needed blinking behavior (Jungmann, R.; Steinhauer, C.; Scheible, M. B.; Kuzyk, A.; Tinnefeld, P.; Simmel, F. C. Single-Molecule Kinetics and Super-Resolution Microscopy by Fluorescence Imaging of Transient Binding on DNA Origami. Nano Lett 2010, 10, 4756–4761). Since dye-labeled oligonucleotides are diffusing in the imaging buffer it is strongly recommended to image the samples in TIRF mode. Epi mode in combination with DNA-PAINT samples would give a very high background noise and thus a very low signal-to-noise ratio.
Usually we recommend using our GATTA-PAINT or GATTA-PAINT HiRes samples in combination with localization based super-resolution microscopes, since this type of sample has a durability of several months, also when prepared ready-to-use (see this link for a one-year-old sample: http://www.gattaquant.com/files/1_year_old_super-resolution_image_of_gatta-paint_80r.png). In case you would like to test your microscope explicitly using the dSTORM mechanism we recommend the STORM samples.
The STORM samples are intended for measurements using the so called dSTORM technique which uses organic dye-molecules as for example Alexa Flour 647 in combination with buffers containing glucose oxidase, catalase and β-mercaptoethanol. See also: Rust, M. J.; Bates, M.; Zhuang, X. Sub-Diffraction-Limit Imaging by Stochastic Optical Reconstruction Microscopy (STORM). Nat Meth 2006, 3, 793–795.
Our STORM and GATTA-PAINT or GATTA-PAINT HiRes samples are both intended to test the achievable spatial resolution of localization based super-resolution microscopes. The difference between the two types of samples is essentially the method of generating the on-off switching of the dye-molecules. Usually we recommend using our GATTA-PAINT or GATTA-PAINT HiRes samples since this type of sample has a durability of several months, also when prepared ready-to-use. Beside the higher sample durability, DNA-PAINT offers higher photostability, higher photon counts and consequently the possibility for higher resolution. In case you would like to test your microscope explicitly using the dSTORM mechanism we recommend the STORM samples. However, since we sell the STORM samples in solution, please note that you have to take care of the surface immobilization and correct buffering.
Additionally to DNA-PAINT nanorulers GATTA-PAINT HiRes samples also contain fiducial markers to use as reference points for drift correction. This tutorial explains how this correction is carried out using our software tool GATTAnalysis.
dSTORM
At each fluorescent mark on the GATTA-STORM ruler we positioned 3-4 Alexa Flour 647 dye-molecules. The DNA origami rulers are very homogeneous with respect to the labeling yield and subsequently also to the number of dye-molecules per fluorescent mark.
For dSTORM we recommend the common buffers based on glucose oxidase, catalase and β-mercaptoethanol, see also: Rust, M. J.; Bates, M.; Zhuang, X. Sub-Diffraction-Limit Imaging by Stochastic Optical Reconstruction Microscopy (STORM). Nat Meth 2006, 3, 793–795.
Software: GATTAnalysis
The licence file might not be recognized correctly, if you start the software out of the zip file or from a network resource. If that is the case, you should extract both files (software and licence) to a local folder on your computer and try to start the software again.
For multicolor samples GATTAnalysis can calculate distances separately for both color channels. This tutorial explains this in detail.
Administrative FAQ
The lead time for our regular products is around 2-3 weeks since we produce the samples on demand. Every probe is handmade and underlies an individual quality control before leaving our lab.
The lead time for custom samples depends on several factors, like lead time of chemicals we need to order, the design complexity and the amount of samples you ordered. Usually custom samples are ready to ship within up to 4 weeks, however in certain cases it might take us longer.
We apologize for delays in the manufacturing process, nevertheless we want to provide you with the highest sample quality possible and if a probe does not attain our quality standards we will not ship it. At the end we want you to have an excellent experimental study, obtaining the best results possible, therefore in our opinion quality is more important than time.
The GATTA-PAINT samples have a durability of more than 1 year under ideal storage conditions. Please have a look at our super-resolution image of a 1-year-old GATTA-PAINT sample: http://www.gattaquant.com/files/1_year_old_super-resolution_image_of_gatta-paint_80r.png
Samples embedded in polymers like our STED, SIM or bead samples have a durability of at least 2 months.
Customer experiences show that the samples can be used for more than 6 months. If you order the samples in solution and freeze them at -20°C, the samples offer a durability of > 1 year.
We ship all of our samples worldwide but we have also distributors in some countries. A list of our current distributors you find here: http://www.gattaquant.com/service/distributors.html
Usually we choose either DHL, TNT/Fedex or UPS.
Yes, that’s possible, also for other services like UPS or DHL.
contact
GATTAquant GmbH
Staffelseestraße 8
DE-81477 München
Phone: +49 89 2153 720 80
INFO@GATTAQUANT.COM
funded by

Payment Options


 English
English German
German Chinese
Chinese